Urdu Translation and Adaptation of Glasgow Antipsychotic Side-Effect Scale (GASS)
1Qambar M. Bokhari, 2Iram Zehra Bokharey*, 3Shahid H. Warris, 4Nauman Mazhar, 5Nadeem Akhtar & 6Irum Fatima
1Omar Hospital & Cardiac Centre, 2Mayo Hospital, 3Services Hospital, 4Punjab Institute of Mental Health, 5Mansoora Teaching Hospital, 6Univeristy of the Punjab
The main objective of the study was “Urdu translation and cultural adaptation” of Glasgow Anti-psychotic Side-effect Scale (GASS). The study comprised two phases and employed a combined methods design. Multiple Forward Translation Method was used to translate and adapt GASS, and a pre-clinical version was administered to 58 indoor patients. Later, psychometric properties and gender patterns of side effects were assessed. The pre-final version of the tool had good content validity with S-CVI of .94 and I-CVIs of .8 to .1. The Cronbach alpha for men indicated good internal consistency and reliability, while it was low for women. Both men and women exhibited mild to moderate severity of symptoms. This study provides a tool to assess side effects of SGAs in our setting.
Keywords: Antipsychotics, Side effects, GASS (Glasgow Antipsychotic side-effect scale)
Our world has become increasingly multicultural and multilingual, in which various methods are employed for decision-making and assessment in clinical, educational, organizational and other areas. As compared to internal medicine, in clinical psychiatry, mental health providers use different methods of assessment which include in-depth interviews, psychological testing, behavioral observation and measuring of physiological parameters. These methods of assessment help in achieving a better knowledge of their patients’ issues (Rose et al., 2018).
One of the methods of clinical assessment is psychological testing. It assesses the patient’s psychological problems, intellectual functioning, personality as well as response and side-effects of various psychotropic drugs used for their treatment. These psychological tests are standardized. This is achieved by testing on a large sample from selected population. It then sets norms and standards, which can differentiate between people having diagnosable psychological problem and people who are free of disease (Morgan & Townsend, 2017).
Since these tests are usually universally applicable, it is less expensive to translate and adapt an existing instrument than to devise a new one for the people belonging to a different culture. Hence, the translation of psychological tests has become widespread as it is less time consuming (Krach et al., 2016). However, it is important to realize that in assessing individuals belonging to different back grounds considerable care is required so that the translation is able to properly convey intended message (Butcher, 2020).
In order to facilitate the translation of psychological tests, the International Test Commission (ITC) has approved a set of guidelines put forward by a group of 12 members selected from different organizations (Gatt et al., 2017). These guidelines aimed at establishing ‘equivalence’ of a test with its translation so that the intended message of a test is carried accurately across the culture. It is also important to recognize that with best translations; psychological instruments that have been validated in a social group might not be able to deliver in some other population set (Epstein et al., 2015). Therefore, to achieve reliable and valid translations, socio-cultural aspects should always be kept in mind (Krach et al., 2016).
Pena (2007) studied equivalence and classified it into four main categories: linguistic, functional, cultural and metric. However, both ITC and Pena do not elaborate on practical steps that need to be adopted systematically to ensure equivalence hence some other, more practical guidelines have to be adopted to guide the translation of psychological tests (Tjosvold, 2017). Historically, translation of psychological tests into different languages aimed to achieve a close linguistic translation, and these involved various forward and backward translation techniques. These techniques were suggested particularly by the American Association of Orthopedic Surgeons (AAOS) (Tjosvold, 2017). However, it may be worthwhile to note that not all items in a psychological test are translatable. Some are poorly translatable while others are totally untranslatable for example, phrases such as “wound up, see the funny side of the things, butterflies in the stomach” as mentioned in The Hospital Anxiety and Depression Scale (Mumford et al., 1991). Thus, many practitioners and testing experts soon realized that a close linguistic translation needs an in-depth explanation of the appropriate translation phrase. Currently, test translators emphasize adaptation instead of close translations. The terms "translation" and “adaptation" are distinct from each other (Hambleton & Li, 2004).
The initial step remains translation, while adaptation is the second step in which the socio-cultural, grammatical, language and the overall background are taken care of (Bolaños-Medina & González-Ruiz, 2013). Adaptation concerning psychological instruments for a different culture is not easy. It should be a carefully thought-out process to keep its utility and reliability at par with the original tool (Cavalcanti et al., 2019). Adaptation of a scale should be carried out keeping in mind the similarities and differences in the background of two cultures, only then it will be useful (Shahnawaz & Malik, 2017). Research can help to further improve our instruments, if we can compare studies done on different population groups (Cavalcanti et al., 2019).
Presently, no single method can be labeled as the gold standard for translation of psychological instruments (Thammaiah et al., 2016). World Health Organization (WHO) guidelines (WHO, 2013), Medical Output Trust recommendations (Tannus et al., 2018) and American Association of Orthopedic Surgeons (AAOS) guidelines are commonly used (Petkovic et al., 2015). AAOS is well-recognized and a very well accepted guideline. It recommends five steps in such a process, namely: (i) Multiple forward translations, (ii) Common Version Synthesis, (iii) Back translations, (iv) Expert Committee Review, (v) Pre-final Testing (Petkovic et al., 2015).
For the last many years schizophrenia is being treated with First Generation Anti-Psychotic Drugs (FGA) which cause high rate of side effects including extra-pyramidal symptoms (EPS) and movement disorders. Nowadays, it is preferred to treat the disease with Second Generation Anti-Psychotic Drugs (SGA) which are less likely to cause EPS and movement disorder, but more likely to be associated with metabolic side effects (Zhao et al., 2016; Hrdlicka & Dudova, 2015).
Clinically it has been observed that the main factor in determining adherence to treatment and the rate of relapse of illness, is the ability to endure adverse reactions of antipsychotic drugs (Prajapati et al., 2019). Hence, there is a dire need to identify and monitor these side effects. There are at least nine side-effect rating scales in English language available at present which can be used for this purpose. Unfortunately, these scales are less relevant because they measure the side effects produced by FGA. Till today, there is a paucity of instruments to measure side effects caused by SGA; one such scale is The Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS). However, it has certain limitations (Morrison et al., 2016).
The Glasgow Antipsychotic Side – Effect Scale (GASS) was developed by Waddell and Taylor (2008) to assess the side effects of SGAs in patients. This scale was developed on a population of 50 patients taking antipsychotics and 50 comparison subjects. It provides a quick, sensitive and reliable method to gauge the side effects as well as their severity. GASS consists of 22 items and the total scores are arbitrarily divided into severity ranges that is, 0–21 = absent/mild side effects; 22–42 = moderate side effects and 43–63 = severe side effects.
GASS showed good discriminatory power and test – retest reliability (.72) (Waddell & Taylor, 2008). In the comparison of GASS with a similar well-established scale (LUNSERS), the K score came out to be 0.73, which indicated good correlation. Test-retest reliability was also acceptable (Huisman et al., 2021). In addition, Bock et al. (2019) find acceptable sensitivity and specificity values and recommend the use of GASS for measurement-based care and decision making.
On account of its utility and efficacy, GASS has been translated and validated in Greece. The internal consistency and interclass correlation coefficient were adequate (Cronbach α = .79 and .96 respectively), while the test - retest reliability was also high (Maria et al., 2014). Furthermore, GASS has also been translated and adapted in Arabic (AlRuthia et al., 2018), Italian (Rodilico et al., 2022) and Japenese (Kitagawa et al., 2020) and found to be an effective, reliable and a patient friendly tool.
To our knowledge no psychometric rating scale is present in the Urdu language for the assessment of side effects of SGAs. Therefore, the present study has been carried out for translation and adaptation of GASS to provide a quick and effective tool, as this would help the clinician to monitor the side effects frequently in order to ensure compliance or alter the dosage/medication.
MethodThis study consists of two phases, the first one is qualitative while the second phase is situated in the quantitative paradigm. The particular design employed was an exploratory sequential mixed methods design, in which the first phase helps inform the second phase (Pardede, 2019).
GASSThe questionnaire considered in this study is Glasgow Anti-Psychotic Side Effect Scale. It assesses side effects of SGAs. It is a self-report side effect scale in English language which is self-explanatory, valid, practical, and informative and takes only 5 minutes to complete. It consists of 22 questions – question number 1-20 are scored on a four-point scale i.e., 0, 1, 2, and 3, where 0 = never and 3 = everyday, while item 21 and 22 are rated on two point scale with 0 = no and 3 = yes. Item 20 and 21 are gender specific. Item 20 is for men only while item 21 is for women only. Therefore, every individual is scored for 21 items. Greater score indicates higher severity of adverse side effects. When the cases were compared with the age matched comparison subjects, the GASS score for both the groups’ differed significantly (Waddell & Taylor, 2008).
Translation and AdaptationFor translation and adaptation of this instrument, AAOS guidelines were used (Petkovic et al., 2015). The process of translation and adaptation entailed the following steps.
Multiple-Forward TranslationThe GASS was given to three psychology interns working at the Department of Psychiatry, Services Hospital, Lahore. All of them held a Master’s degree in Psychology. They were chosen because their mother tongue was Urdu, which is the primary language of the target culture. Moreover, they also seemed to be acquainted with the English-speaking culture. Before they were given the tool, they were informed that the aim of the translation was a conceptual equivalence of a word or phrase, rather than a “word –for –word” translation. Moreover, the study aimed for the clinical population, which more often than not, tends to be not so educated; hence the translation should be simple, clear and concise. Last but not the least, the translators were also instructed to avoid technical jargon or phrases which the target population might find difficult to comprehend. It took about a month for this process to be completed.
Common Version SynthesisThe study hypothesized that adolescents experiencing emotional difficulties (alexithymia) would likely experience greater psychological distress compared to without alexithymia adolescents, and the results confirmed this hypothesis. Table 4 demonstrates that adolescents with alexithymia had elevated levels of depression, anxiety, and stress, while non-alexithymic adolescents reported lower levels of these psychological difficulties. Several theoretical models have proposed explanations for the relationship between alexithymia and psychological distress. One model suggests that alexithymia impairs effective emotion regulation, leading to negative emotions such as depression, anxiety, and stress (Moriguchi & Komaki, 2013). Another model suggests that alexithymia disrupts interpersonal relationships, resulting in a lack of social support and poorer mental health outcomes (Kooiman et al., 2004). These findings align with previous research conducted by De Berardis et al. (2021), Bhaskar et al. (2021), and Farina et al. (2021), which also found higher levels of depression and anxiety among alexithymic adolescents compared to non-alexithymic adolescents. Moreover, Farina et al. (2021) demonstrated that alexithymic adolescents experienced a range of psychopathological symptoms, including anxiety, depression, and interpersonal hypersensitivity.
The study hypothesis proposed that "Adolescents with alexithymia are expected to have higher levels of parental bonding (specifically, overprotection) compared to non-alexithymic adolescents" in terms of group differences. The results of the study support this hypothesis, as indicated in Table 5. Previous research has also found that individuals with alexithymia tend to experience lower levels of nurturance and higher levels of overprotection from their caregivers, which aligns with the current findings. Excessive parental protectiveness can instill a sense of incompetence in children, and its adverse effects on their development can contribute to emotional challenges (Tolmunen et al., 2011). Alexithymic adolescents reported increased degrees of despair, anxiety, stress, and perceived overprotection from their carers. On the other hand, non-alexithymic adolescents reported minimal or no such issues, as shown in Table 5 (Ahmed et al., 2021). The difficulty of alexithymic individuals in expressing and understanding their emotions leads to communication challenges and hindered personality development, thus exacerbating psychological problems such as anxiety, depression, and stress. Additionally, recent studies have indicated that overprotective parents can be perceived as controlling, intrusive, and authoritarian, negatively impacting the personality development of adolescents. Nevertheless, it is crucial to acknowledge the limitations of this study, which comprise its cross-sectional design and the restricted age range of 16 to 21 years, focusing on middle and late adolescents. To obtain a more comprehensive understanding of the subject, future research endeavors should adopt a longitudinal design and encompass a broader age spectrum.
Common Version Synthesis
In this step, a bilingual expert panel comprising two psychiatrists and one clinical psychologist went through all the three forward translations item by item and came up with a Common Urdu Version (CUV) of the three forward translations. The goal was to identify the best translation not only for each item of the questionnaire but also for the accompanying instructions for scoring the items. In case where it seemed that all the three translations did not adequately reflect the original item, alternatives and modifications of the items were suggested. All in all, eight meetings were held to finalize the CUV synthesis over a period of two months.
Backward Translation
As in the first step, the CUV of the instrument was given to three bilingual experts, lecturers in a local college, who were not related to the research group. Furthermore, they had no idea of the tool as well. Their task was to translate the CUV back into the English language. This process helped arrive at a translated Urdu tool which conveyed similar meanings when administered on the native population.
Expert Committee ReviewIn this step, the expert panel of step two compared and analyzed the CUV and backward translations. Again, the modification and rephrasing of items of CUV were undertaken whenever required, to produce the pre-final version. The aim of this step was to ascertain whether the translation was accurate and truly reflected the intent of the original item. Four meetings were held to finalize the tool for the next step.
Pre-final TestingThis stage is also called the cognitive interviewing/debriefing and involves administering the pre – final version of the tool on the target population. Thus, the tool was administered to 76 admitted patients with schizophrenia at the Department of Psychiatry, Services Hospital, Lahore. However, only 58 responded to all items. The remaining either had issues with attention or did not understand Urdu. Therefore, further analyses were done only on these 58 participants. All of these patients were taking various SGAs in optimum dosages. The rationale of this phase was to obtain the feedback of the patients regarding their comprehension. It enabled us to verify language proficiency and cultural adaptation. However, none of the items in the final version posed any difficulty for the participants; therefore, all of them were retained. The final Urdu version of the tool generated during phase 1 is provided in Table 1.
ParticipantsThe sample comprised 58 patients admitted in the Department of Psychiatry, Services Hospital, Lahore, Pakistan, chosen through purposive sampling. There were 42 men with an age range between 15 to 59 years (M =33.55, SD= 10.49) and 16 women with an age range between 23 to 42 years (M = 30.68 and SD =.5.59). Inclusion Criteria: The inpatients diagnosed with schizophrenia (fulfilling the DSM – V criterion) were included in the study. The minimum age was 15 years and the patients had been on optimum dosage of various SGAs for at least one month. Exclusion Criteria: The patients with dual diagnoses were excluded. The study was approved by the Institutional Review Board of Services Institute of Medical Sciences, Lahore, Pakistan. Moreover, a written informed consent was taken from all the participants. Descriptive statistics were used to calculate the frequencies and percentages. Mean and standard deviation for age were determined, while Cronbach alpha was used to calculate internal consistency. Content validity was also determined by calculating item content validity index as well as scale content validity index.
Results
ReliabilityIn order to run reliability analysis through Chronbach Alpha it was assessed if the sample was sufficient for this analysis. Following Bonett (2002), and Bujang, Omar and Baharum (2018), the minimum sample size of 14 was determined for 21 items with the power of .80, alpha of .05 (two tailed), and reference Chronbach Alpha of .70. It was observed that the number of men and women in current study was sufficient to run Chronbach Alpha. In GASS as one item was different for men and women, internal consistency was checked for men and women separately.
Table 2
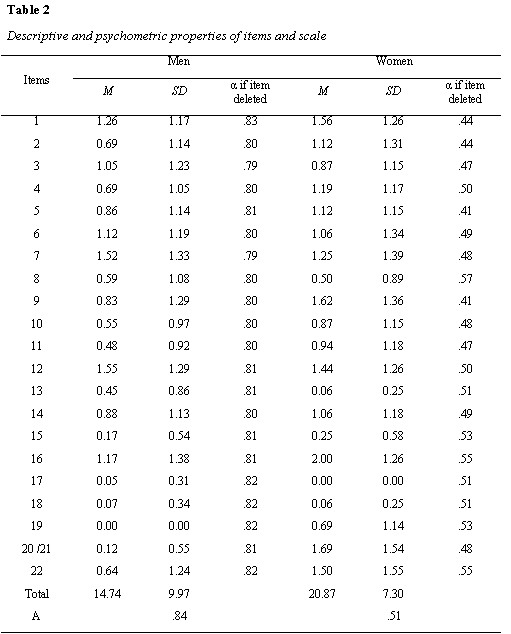
In table 2, Mean, Standard deviation, Item total correlations (rit), Cronbach alpha, if the item deleted along with Cronbach alpha for the full scale have been presented for men and women separately. It can be observed that internal consistency of the scale for men is good, while for women it is not satisfactory. For men Cronbach alpha deleted for all items is less than overall Cronbach alpha of the scale, showing that deleting those items will reduce the Cronbach alpha. For women deleting items, 8, 15, 16, 19 and 22, there is minor increase in Cronbach alpha which does not justify deletion of these items from the scale. Further examination of item characteristics shows that men had zero mean and standard deviation on item 19 (I had problem enjoying sex), while women had zero mean and standard deviation on item 17 (The areas around my nipples had been sore and swollen). This means that these side effects were not observed in men and women in the current study respectively.
Content ValidityTo establish the content validity of the translated tool, five psychiatrists with a minimum experience of 10 years of clinical practice were asked to rate each item on a 4 point scale (1= not relevant, 2 = slightly relevant, 3 = very relevant and 4= most relevant). First of all the, item content validity Index (I-CVI) was calculated for each item as the number of experts giving a rating of either 3 or 4, divided by the total number of experts. Secondly, Scale content validity index (S-CVI) was calculated by averaging of I-CVIs (Polit & Beck, 2006). The scale had good content validity (Waltz et al, 2005) with S-CVI of .94 (I-CVIs = .8 to .1) (AlRuthia et al., 2018; Polit & Beck, 2006).
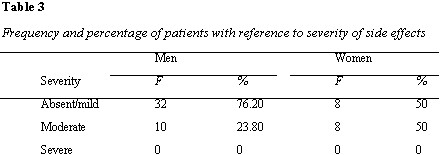
Analyzing at the means of total side effects it can be seen that side effects reported by patients in the current study are generally mild. Furthermore, women reported more side effects than men, t (56) = 2.24, p = .03. The sample was also categorized in terms of absent/mild, moderate and severe side effects (Waddell and Taylor, 2008). Distribution of severity of side effects in men and women (table 3) shows that both men and women report absent/mild to moderate symptoms with equal distribution of absent/mild and moderate in women. On the other hand, more absent/mild symptoms were observed in men, than moderate symptoms. Neither men nor women showed severe symptoms.
This study presents the first translation of GASS into Urdu, the national language of Pakistan. The reason for choosing this tool was that it is an easy to administer and patient friendly instrument. The final sample size (n=58), although small, was found to be statistically significant to run the analyses (Bonett 2002; Bujang et al., 2018). As one item was different for men and women, the internal consistency was checked for men and women separately (Table 2). The internal consistency for men was good and ranged from .79 to .83. On the other hand, the internal consistency for women was not satisfactory as it ranged from .41 to .57. The reason could be that most of the women participants hailed from rural areas and the mental health literacy of women from these areas is often compromised. Moreover, they tend to be socially disinhibited, and not likely to express themselves openly and perhaps were less likely to express side effects (AlRuthia et al., 2018). The lack of mental health literacy and stigma in developing countries such as Pakistan and different cultural beliefs associated with mental health, could also account for this (Munawar et al., 2020).
Moreover, the gender distribution of side effects shows that they fell in the absent/mild or moderate category. The scale content validity index (S-CVI) came out to be .94, which is quite high (Waltz et al., 2010; Polit & Beck, 2006). Furthermore, men had zero mean and standard deviation on item 19 (I had problem enjoying sex) while women had zero mean and standard deviation on item 17 (The areas around my nipples had been sore and swollen). There could be a two-pronged explanation for this. First of all, this could point towards the absence of these side effects in men and women. The second reason could be that talking about sexual issues; even with health professionals is not considered acceptable by most of the population seeking treatment in government run health facilities on account of social inhibitions and lack of awareness.
Regarding the severity of side effects, both men and women reported absent/mild to moderate symptoms with an equal distribution (50% each) in women. On the other hand, more absent/mild symptoms (76.20%) were observed in men than moderate (23.80%) symptoms. Neither men nor women exhibited any severe symptoms. Our findings are in contrast to previous literature, where women experienced more side effects of anti-psychotic medication as compared to men (AlRuthia et al., 2018; Seeman, 2009). More studies need to be carried out for an in – depth exploration of this difference.
This study has few limitations. First of all, on account of low reliability regarding women, this translated tool is not currently suitable to be used for the women population. More studies need to be conducted to see if the pattern is the same or different in other women samples. Secondly, test-retest reliability could not be done as it was difficult to interview the same participant for the second time due to early discharge and inability of most patients to maintain follow-up. Thirdly, it would have been more appropriate to take larger samples with equal gender distribution in order to draw more comparisons regarding more variables such as the type of specific second-generation antipsychotic, severity of symptoms, length of illness etc.
Conclusion and RecommendationThis study has provided a useful, quick and simple tool to assess the side effects of SGAs at a time when there seems to be no such tool available in Urdu language currently. Furthermore, gender difference in reliability and side effects highlight the importance of evaluating these separately for men and women on larger samples in future studies. Lastly, the detailed description of the entire procedure can be of help to researchers involved in similar projects.
References
AlRuthia, Y., Alkofide, H., Alosaimi, F. D., Alkadi, H., Alnasser, A., Aldahash, A., Basalamah, A., & Alarfaj, M. (2018). Translation and cultural adaptation of Glasgow antipsychotic side-effects scale (GASS) in Arabic. PLOS ONE, 13(8), e0201225. https://doi.org/10.1371/journal.pone.0201225
Bock, M. S., Van Achter, O. N., Dines, D., Correll, C. U., Mors, O., Østergaard, S. D., & Kølbæk, P. (2019). F28. Clinical validation of the Glasgow antipsychotic side effect scale (Gass). Schizophrenia Bulletin , 45(Supplement_2), S265. http://doi.org/10.1093/sbz018.440/
Bolaños-Medina, A., & González-Ruiz, V. (2013). Deconstructing the translation of psychological tests. Meta, 57(3), 715-739. https://doi.org/10.7202/1017088ar
Bonett, D. G. (2002). Sample size requirements for testing and estimating coefficient Alpha. Journal of Educational and Behavioral Statistics, 27(4), 335-340. https://doi.org/10.3102/10769986027004335
Bujang, M. A., Omar, E. D., & Baharum, N. A. (2018). A review on sample size determination for Cronbach’s Alpha test: A simple guide for researchers. Malaysian Journal of Medical Sciences, 25(6), 85-99. https://doi.org/10.21315/mjms2018.25.6.9
Butcher, J. N. (2020). Fifty Historical highlights in cross-cultural MMPI/MMPI-2/MMPI-A assessment. Minnesota Multiphasic Personality Inventory (MMPI).https://mmpi.umn.edu/sites/mmpi.umn.edu/files/2022-05/highlights_in_cross-cultural_mmpi_assessment-2020_1.pdf
Cavalcanti, A. D., Pinto, K. D., & Maia, E. M. (2019). Adaptação transcultural para o português do instrumento patient dignity inventory. Revista de Enfermagem UFPE on line, 13(3), 879. https://doi.org/10.5205/1981-8963-v13i3a238808p879-883-2019
Epstein, J., Santo, R. M., & Guillemin, F. (2015). A review of guidelines for cross-cultural adaptation of questionnaires could not bring out a consensus. Journal of Clinical Epidemiology, 68(4), 435-441. https://doi.org/10.1016/j.jclinepi.2014.11.021
Gatt, I., West, L. M., Calleja, N., Briffa, C., & Cordina, M. (2017). Psychometric properties of the belief about medicines questionnaire (BMQ) in the Maltese language. Pharmacy Practice, 15(1), 886-886. https://doi.org/10.18549/pharmpract.2017.01.886
Hambleton, R. K., & Li, S. (2004). Statistical methods for identifying flaws in the test adaptation process. Adapting Educational and Psychological Tests for Cross-Cultural Assessment, 105-128. https://doi.org/10.4324/9781410611758-9
Hrdlicka, M., & Dudova, I. (2015). Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatric Disease and Treatment, 907. https://doi.org/10.2147/ndt.s82185
Huisman, R., Okhuijsen-Pfeifer, C., Mulder, E. Y. H., Jongkind, A., Cohen, D., Bogers, J. P. A. M., Van der Horst, M. Z., & Luykx, J. J. (2021). Validation of the Dutch Glasgow Anti- psychotic Side-Effect Scale for Clozapine. Tijdschr Psychiatr, 63(4), 270-275.
Kitagawa, K., So, R., Nomura, N., Mizuno, Y., Misawa, F., Kodama, M., Uchida, H., Mimura, M., & Takeuchi, H. (2020). Reliability of the Glasgow antipsychotic side-effects scale for Clozapine Japanese version (GASS-C-J). PLOS ONE, 15(6), e0234864. https://doi.org/10.1371/journal.pone.0234864